News
bioMérieux, a world leader in the field of in vitro diagnostics, has finalized the closing of the acquisition of Neoprospecta on January 29th, 2025. A Sale and Purchase Agreement (SPA) to acquire this Brasilian-based company was signed in November 2024. Neoprospecta, based in Florianopolis, develops and markets innovative user-friendly data and genomics solutions for augmenting quality assurance programs and improve microbiological risk prevention in food and pharma industries.

Swipe to discover more
-
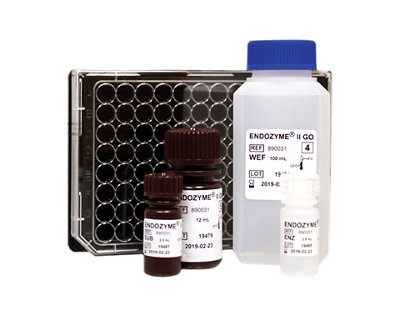
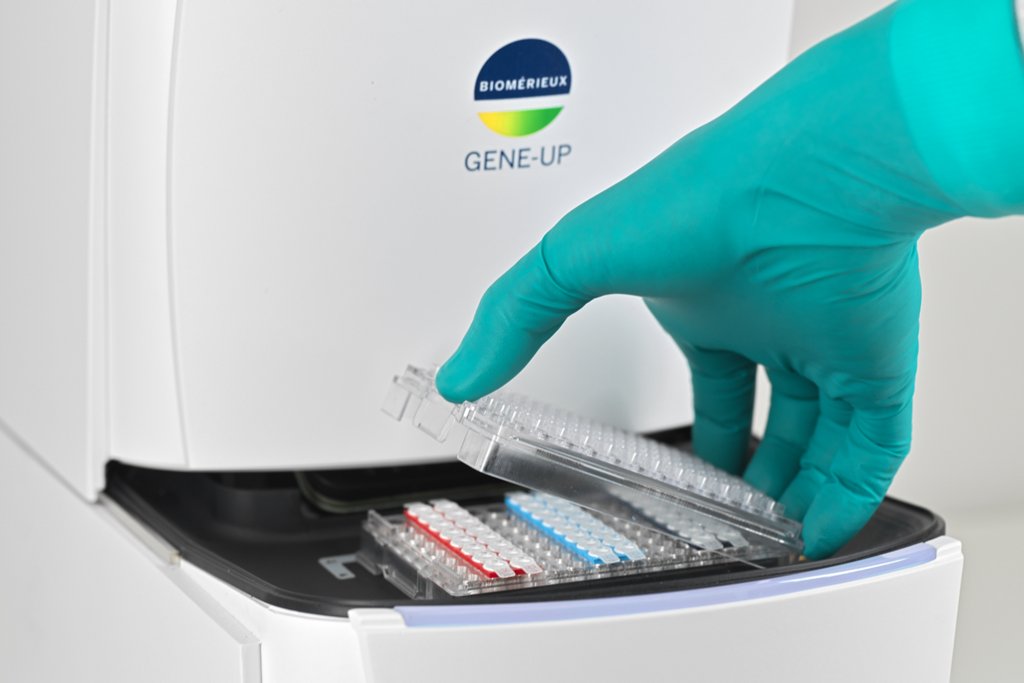
bioMérieux launches GENE-UP® TYPER, an innovative diagnostic solution for food industries to rapidly analyze the root cause of contamination of Listeria monocytogenes
bioMérieux, a world leader in the field of in vitro diagnostics, today announces the launch of GENE-UP® TYPER, a real-time PCR solution for rapid root cause analysis in the food industry. -
Clinical Informatics & Artificial Intelligence Can Help Advance Antimicrobial Stewardship
Worldwide escalation in antimicrobial resistance (AMR) has sparked fear of a looming post-antimicrobial era. Antibiotic overuse and inappropriate use—including over-prescribing, inaccurate, and suboptimal prescribing—promotes bacterial evolution and resistance to treatment. Data suggests that there were an estimated 4.95 million deaths associated with bacterial AMR in 2019. The World Bank estimates that AMR could result in 1 trillion dollars in additional healthcare costs by 2050 if actions are not taken now to curb growing resistance. -
With E-Resale, bioMérieux innovates by giving a second life to its instruments
Launched last July, starting with Africa and Middle East, the E-Resale platform allows to purchase and sell second-hand bioMérieux instruments for clinical applications. This is an innovative offer in the field of circular economy and a first on the in vitro diagnostics market.
Enabling Decision-Making
- ANTIMICROBIAL STEWARDSHIP
- SEPSIS
- FOOD SAFETY & QUALITY
- PHARMA QUALITY CONTROL
- COVID-19
- ANTIMICROBIAL STEWARDSHIP
- SEPSIS
- FOOD SAFETY & QUALITY
- PHARMA QUALITY CONTROL
- COVID-19













?qlt=85&ts=1670238196189&dpr=off)