
2024
2024
2024
bioMérieux announces the acquisition of LUMED, a software company that has developed a clinical decision support system to help hospitals optimize antimicrobial prescriptions and monitor healthcare-associated infections.
bioMérieux was created in 1963, but our roots go all the way back to the 19th century, with Marcel Mérieux, a student of Louis Pasteur.
Have a look at the key milestone that have forged our entrepreneurial journey!

bioMérieux announces the acquisition of LUMED, a software company that has developed a clinical decision support system to help hospitals optimize antimicrobial prescriptions and monitor healthcare-associated infections.

bioMérieux made an immediate £70M investment in Oxford Nanopore, the company delivering a new generation of nanopore-based molecular sensing technology.
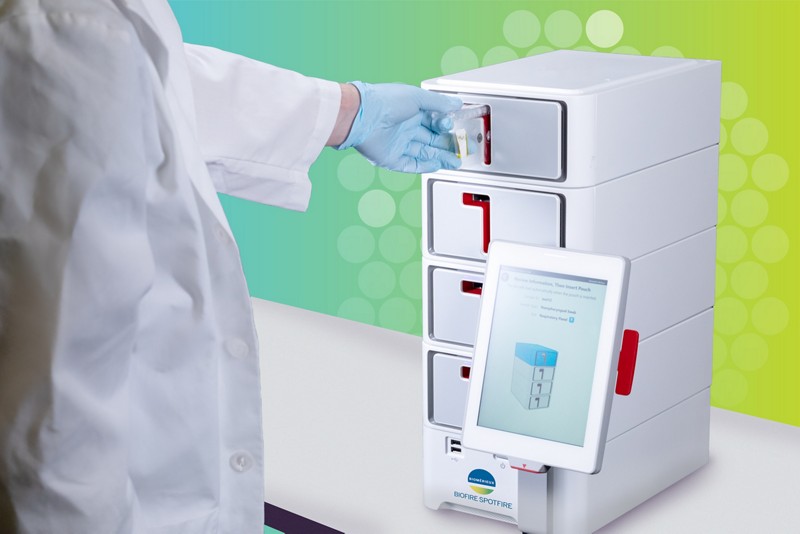
The BIOFIRE® SPOTFIRE® System received FDA 510(k) Clearance and CLIA-Waiver. This fast and innovative PCR system can be used by non-lab professionals at the point-of-care and delivers results in approximately 15 minutes.

bioMérieux evolves its governance: Alexandre Mérieux becomes Executive President and Pierre Boulud is appointed Chief Executive Officer. This evolution in governance separates the functions of Chairman and Chief Executive Officer. Pierre Boulud will directly lead the Executive Committee of bioMérieux to implement the Company’s strategy. In the face of major changes in the global healthcare sector, Alexandre Mérieux will focus on key issues related to global strategy.

FDA 510(k) clearance for VITEK® MS PRIME, the new MALDI-TOF mass spectrometry identification system of bioMérieux.

bioMérieux acquired Specific Diagnostics, a privately held U.S. based company that has developed a rapid antimicrobial susceptibility test (AST) system that delivers phenotypic AST directly from positive blood cultures.
A joint venture to fight AMR: Boehringer Ingelheim, Evotec SE and bioMérieux announced that they have formed a joint venture to create the next generation of antimicrobials along with actionable diagnostics.

CE-marking of VIDAS® KUBE™, a new generation automated immunoassay system for the renowned VIDAS® range. This innovation benefits clinical labs and food industries by providing results to help speed up patient care and protect consumers.

Co-exclusive distribution agreement with Specific Diagnostics
bioMérieux distributes Specific Diagnostic’s newly introduced SPECIFIC REVEAL® Rapid AST in Europe, where it has been CE-IVD approved.
This system provides actionable results for bloodstream infections in an average of 5 hours directly from positive blood culture.

CE marking of VIDAS® DENGUE NS1 Ag, VIDAS® Anti-DENGUE IgM and VIDAS® Anti-DENGUE IgG tests
Innovative and fully-automated assays to diagnose dengue infection.
Can be used independently to detect the viral antigen (NS1) and antibodies (IgM and IgG).

CE marking of VIDAS® NEPHROCHECK® test
Innovative test to detect kidney stress in patients at risk of acute renal failure (AKI).
Using VIDAS® 3 system.
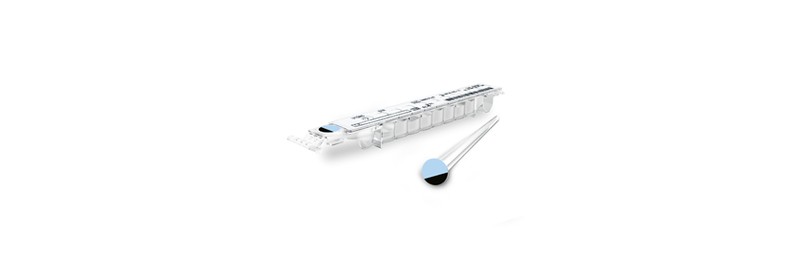
CE marking of TB-IGRA® test on VIDAS®
Innovative and fully-automated test (Interferon-Gamma Release Assay) to diagnose latent TB infection.
Performed on the VIDAS® 3 platform.

Launch of BIOFIRE® MYCOPLASMA
Molecular biology test for mycoplasma detection in biopharmaceutical products.

CE marking of SARS-COV-2 RESPI R-GENE® test
Molecular biology test in the ARGENE® range for the detection of SARS-CoV-2, influenza viruses A and B, RSV (human respiratory syncytial virus) and hMPV (human metapneumovirus) from a single sample.

CE marking of VIDAS® SARS-COV-2 IgM and SARS-COV-2 IgG
Serology tests used to measure the presence of antibodies in people who have been infected with SARS-CoV-2.

Launch of the SARS-COV-2 R-GENE® test
ARGENE® range test for the detection of SARS-CoV-2 coronavirus.

EUA for the BIOFIRE® RP2.1 panel and CE-marked of the BIOFIRE® RP2.1plus panel
Emergency use authorization (EUA) from the FDA for the BIOFIRE® RP2.1 panel covering 22 pathogens responsible for respiratory infections, including SARS-CoV-2.
The CE-marked BIOFIRE® RP2.1plus panel also detects MERS-Coronavirus in addition to the SARS-CoV-2 virus.

BIOFIRE® COVID-19 test
Emergency Use Authorization by the US Food and Drug Administration (FDA) of the BIOFIRE® COVID-19 test for the detection of SARS-CoV-2.

Acquistion of Invisible Sentinel (United-States)
A company specialized in user-friendly molecular diagnostic tools for the rapid, accurate and reliable detection of pathogens and spoilage organisms in food and beverage.

CE mark for expanded VITEK® MS database
The latest additions to the database used by this automated mass spectrometry microbial identification system allows the identification of :
This innovative solution further improves the performance of the VITEK® MS system by adding 272 new species to its database, including 217 bacteria species and 55 fungal species.

Acquisition of a majority stake in Hybiome (China)
The Chinese Company Suzhou Hybiome Biomedical Engineering Co.Ltd is specialized in automated immunoassay tests.
Founded in 2009, the company develops, manufactures and markets a complete range of diagnostic solutions (reagents, instruments and software) cleared by the China Food and Drug Administration (CFDA).

Launch of the BIOFIRE® FILMARRAY® Pneumonia Panels
The BIOFIRE® FILMARRAY® Pneumonia panel received 510 (k) clearance from the Food and Drug Administration (FDA).
The BIOFIRE® FILMARRAY® Pneumonia Panel plus received the CE-Mark.
These panels aid in the diagnosis of lower respiratory tract infections.

Acquisition of Astute Medical (United-States)
Dedicated to improving the diagnosis of high-risk medical conditions and diseases through the identification and validation of protein biomarkers.
Astute developed the NEPHROCHECK® test, an FDA-cleared test for the early risk assessment of acute kidney injuries (AKI).
This acquisition follows a development and commercialization partnership signed in 2015 with Astute to develop and market the NEPHROCHECK® test, for the VIDAS® automated immunoassay system. It reinforces bioMérieux’s offering of High Medical Value immunoassay biomarkers.
Fruitful partnership developed in 2015 between Astute Medical and bioMérieux when Astute granted bioMérieux a license to develop and market the NEPHROCHECK® test for the VIDAS® automated immunoassay system
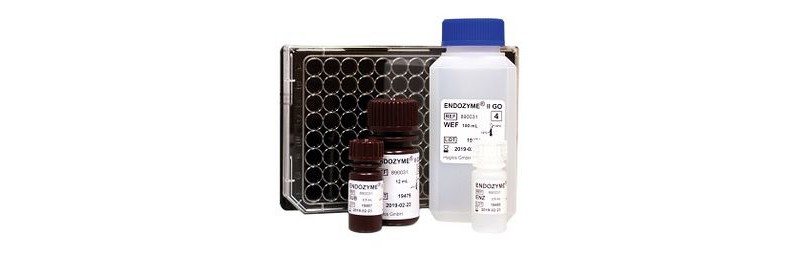
ENDOZYME® II GO launch

FDA clearance for expanded pathogen identification capability on VITEK® MS
VITEK® MS, rapid pathogen identification, has received 510(k) clearance from the FDA for the expanded identification of mycobacteria, Nocardia and moulds.

FDA 510(k) clearance for the BacT/ALERT® VIRTUO™ automated blood culture system
BacT/ALERT® VIRTUO™ is the first continuously-monitoring blood culture microbial detection system to offer “Load & Go” technology, helping labs to streamline their workflow. It is now commercially available in countries that recognize CE marking and in the United States.
FDA clearance for RAPIDEC® CARBA NP test
This test was developed for cases where microorganisms are resistant to carbapenems (a very broad spectrum antibiotic class).
Il enables the detection of carbapenemase producers within 2 hours.

Alexandre Mérieux appointed Chairman and CEO of bioMérieux
After serving as CEO of bioMérieux for the last three years, Alexandre Mérieux succeeds Jean-Luc Bélingard, who had been Chairman of the Company since 2010.
As the next Mérieux generation takes up the position of Chairman and CEO, this appointment marks the continuity of the family’s role in the Company.

FDA clearance for VIDAS® B•R•A•H•M•S PCTTM
This automated test for the measurement of procalcitonin (PCT), a biological marker of bacterial infections, is an aid for antibiotic stewardship in respiratory infections and sepsis.
It provides test results in 20 minutes.

FDA clearance for BIOFIRE® FILMARRAY® RP2, CE marked for RP2 plus
The Respiratory BIOFIRE® FILMARRAY® 2 (RP2) panel, enriched version of the Respiratory BIOFIRE® FILMARRAY® panel, tests in 45 minutes 21 pathogens (17 viruses and 4 bacteria) responsible for respiratory tract infections. It incorporates an additional pathogen, Bordetella parapertussis, one of the causative agents of pertussis or whooping cough.
The BIOFIRE® FILMARRAY® RP2 plus includes one additional pathogen: Middle East Respiratory Syndrome coronavirus (MERS-CoV).

Launch of EVISIGHT™ COMPACT
A new automated diagnostic solution for microbial detection in pharmaceutical production and an intelligent incubator system providing real time culture media reading.

Launch of EMAG®
A new molecular biology platform for the extraction of nucleic acids (DNA, RNA) that builds on the quality, robustness and ease of use that have made the NucliSENS® easyMAG® platform so successful. eMAG® features automation from the primary sample tube, greater traceability and higher throughput, in addition to an unparalleled degree of flexibility, not previously available on an automated system for the extraction of nucleic acids.

Acquisition of Applied Maths (Belgium)
bioMérieux enhances its bioinformatics capabilities with cutting-edge expertise and solutions for the smart use of complex biological data.

FilmArray® Torch is FDA cleared with all 4 existing FilmArray® Panels and the system gets CE marked
The FilmArray® Torch platform is FDA-cleared for use with the Respiratory, Blood Culture Indentification, Gastrointestinal and Meningitis/Encephalitis panels. It provides up to six times more sample throughput per square foot of benchtop space.

Acquisition of Hyglos (Germany)
Acquisition of Hyglos, a company that brings bioMérieux a unique and recognized expertise in the development and production of recombinant proteins used for the detection of endotoxins in pharmaceutical products.

Moving of our headquarters to the Campus de l'Étoile
bioMérieux's Corporate functions are grouped on a new building in Marcy l’Étoile.(Lyon - France).
It is located at the roots of the Company's geographical center.
The historic site in Marcy concentrates on production, quality and R&D activities.

Strategic partnership between bioMérieux and COPAN (Italia)
Strategic partnership in clinical microbiology laboratory automation between bioMérieux and COPAN. Leading manufacturer of innovative pre-analytic solutions, COPAN grants distribution rights for its automated platforms WASP® and WASPLab™.

Launch of GENE-UP®
A new-generation PCR system for customers in the agri-food sector for the detection of microorganisms (bacteria and viruses).

Launch of bioMérieux EPISEQ™
An innovative next-generation sequencing (NGS) service dedicated to the epidemiological monitoring of bacterial infections. Result of the collaboration agreement with Illumina, a world leader un genomics.

Launch of CE-marked VIRTUO™
The new generation of BacT/ALERT®, VIRTUO™ is a uniquely innovative automated blood culture microbial detection system.

Acquisition of BioFire Diagnostics Inc.
Acquisition of BioFire Diagnostics Inc., a privately held US-based company specialized in molecular biology.

FDA clearance for the FilmArray® Gastrointestinal (GI) Panel
The 22-target FilmArray® GI Panel1 allows a syndromic approach2 to the diagnosis of infectious diarrhea as it includes bacteria, viruses and parasites in one test.
A test panel is a predetermined group of medical tests used as an aid in the diagnosis and treatment of diseases.
The syndromic approach is based on analyzing a syndrome (i.e. a set of symptoms) and, with a single reagent, identifying the disease-causing organisms responsible for this syndrome, whether they are viruses, bacteria or parasites.

VIDAS® 3
The new generation of the VIDAS automated immunoassay platform.
FDA approval of novel molecular test THxID™-BRAF
A companion test for late stage metastatic melanoma tumor samples.

Acquisition of RAS (India) and partnership with Quanterix

Launch of VITEK® MS

Acquisition of AES Laboratoire (France).
An industrial microbiology company.

Acquisition of ARGENE (France)
A specialist in molecular biology.

Launch of Myla® middleware

Acquisition of Meikang Biotech and of Shanghai Zenka Biotechnology.
Acquisition of Meikang Biotech (China), a manufacturer of rapid tests.
Acquisition of Shanghai Zenka Biotechnology (China), a specialist in culture media.

Launch of PREVI® Color Gram
PREVI® Color Gram for automated Gram staining.

Joint ventures and Acquisitions

Acquisition of Biomedics (Spain) and of BTF (Australia)

Acquisition of Bacterial Barcodes (USA)

Launches of TEMPO® and VITEK® 2 Compact
TEMPO®, for automated enumeration of quality indicators (bacteria, yeast and mold) for the agri-food industry.
VITEK® 2 Compact, a system for identification and antibiotic susceptibility testing (AST).

bioMérieux is listed on the stock exchange.

NucliSENS EasyQ® and NucliSENS EasyQ® HIV-1
NucliSENS EasyQ®, a molecular diagnostics platform for amplification and real-time detection.
NucliSENS EasyQ® HIV-1, the first real-time test for measuring HIV-1 viral load.

Acquisition of OrganonTeknika (Akzo Nobel, Netherlands).
A pioneering company working in HIV screening and viral load measurement.

Launches of BacT/ALERT® 3D and VITEK® 2
*Organon Teknika was acquired by bioMérieux in 2001.
VIDAS® D-Dimer Exclusion™
The first test used to rule out the diagnosis of deep vein thrombosis and pulmonary embolism to be certified by the FDA.

VIDAS® automated immunoassay platform adapted for industrial applications.

Launch of the VIDAS® with 8 initial kits Today VIDAS has 100 parameters.

Acquisition of the BOOM extraction and NASBA amplification technologies for molecular diagnostics.
Development of chromogenic media Culture media that enables identification of microbial colonies by coloring them.

Acquisition of Vitek Systems (United States) A world leader in automated bacterial identification.

Acquisition of Api Systems (La Balme, France).
A specialist in bacterial identification and antibiotic susceptibility testing systems.
ATB™ EXPRESSION™ et ATB™ Plus Expert for automated bacterial identification and antibiotic susceptibility testing
First HIV screening test: VIRONOSTIKA® HIV anti-HTLV-III by Organon Teknika*
*Organon Teknika was acquired by bioMérieux in 2001

ATB™ strip
First ATB™ Antibiotic Susceptibility test strip by Api System*
*Api System was acquired by bioMérieux in 1987

Launch of the Slidex® Meningitis kit.
First test enabling the diagnosis of an infectious disease without prior culture, with results in 5 minutes compared to several days previously.

Alain Mérieux takes control of BD Mérieux, which becomes bioMérieux.

Production and sale of the first ready-to-use culture media in Petri plates.

The first toxoplasmosis detection kit and launch of miniaturized API® 20E strip
*Api System was acquired by bioMérieux in 1987.

Launch by BD Mérieux of a fibrometer
The fibrometer is added to the BD Mérieux catalog to test blood coagulation and is quickly adopted by a quarter (approx. 1,000) of France’s laboratories.

Establishment of BD Mérieux