SCANRDI®
Bring Your Sterility Testing to the Next Level
SCANRDI® revolutionized rapid microbial detection when it was first introduced and its speed and sensitivity continue to answer market needs today.
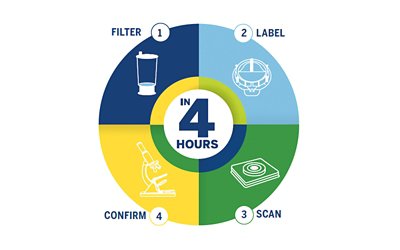
Its unique technology delivers actionable sterility results in 4 hours or less, so you know you can continue with production or if you need to take action immediately.


- Overview
- Technology
- Services
- Resources
When You Need to Know Now
SCANRDI® enables same-shift detection of microbial contaminants in filterable drug products. The US Food and Drug Administration (FDA), European Medicines Agency (EMA), and other regulatory bodies support the use of validated Rapid Microbiological Methods (RMM) for sterile product release - especially in the case of shelf-life products, where a rapid result is more suitable than a compendial 14-day test.




- Filter
- Label
- Scan
- Confirm
Enhanced Device Functionality
NEW! Combine your SCANRDI® instrument with SCANFILTERTM, our new filtration device.
- Enhanced user-friendliness, encompassing streamlined handling, optimal sample visibility, and effortless packaging access
- Improved robustness with high-quality materials to minimize operator errors
- Enhanced user experience facilitated by the device, necessitating minimal manipulation with heightened precision
- Enhanced device functionality leads to increased workflow efficiency and reduced error risk
Improve Productivity and Compliance
Increase Your Market Competitiveness
- Safely release your short shelf-life & generic products faster
- Optimize your distribution logistics with just-in-time inventory management of stock and choose your most affordable shipping option
Control Your Manufacturing Process
- Rapid in-process, bioburden and sterility results give you confidence that your production is operating properly
- Facilitate your investigation process to quickly return to normal operations
- Eliminate lab bottlenecks
Be Confident in Your Compliance
- Ensure your electronic compliance with 21 CFR part 11 enabled software, barcode scanning traceability, several approval levels and a full audit trail
- Feel reassured in your validation process with support from bioMérieux including URS completion guide, IOQ guide, primary validation guide, suitability guide and Drug Master File (DMF)
Ultra-rapid
- Same-shift results
- Release your product faster
Accurate
- Tested & proven technology
- Make decisions with confidence
Compliant
- Full validation support: URS completion guide, IOQ guide, primary validation guide, suitability guide and Drug Master File (DMF)
- 21 CFR part 11 enabled software
Proven Technology
- Long history of use for final product sterility, in-process bioburden control & water testing
- Down to 1 microbe/filtrable volume
- >200 compatible products
Broad Specificity
- Universal fluorescent labeling
- Bacteria, yeast, and mold detection
More Than 200 Compatible Products*
- Antibiotics
- Isotonic solutions
- Chemotherapy drugs
- Hormones
- Anti-inflammatories
- Anti-coagulants
- Analgesics
- Diuretics
- Vitamins
* non-exhaustive list
SCANRDI® is a rapid, proprietary microbiological solution combining universal cell labeling and solid-phase cytometry with the ability to detect bacteria, yeasts, molds – both in vegetative and sporulated forms - and yeasts. Test applications include:
- Raw material testing
- Water testing
- In-process product testing
- Chromatography columns health check
- Investigations
- Product release
As it’s a non-growth based method, stressed and fastidious organisms including those in VBNC state (Viable but Non-culturable) can be detected as well, making SCANRDI® a powerful tool to monitor and ensure the rapid detection of microorganisms in pharmaceutical products.
Services: Feasibility, Validation, and Instrument Support
Your productivity and product quality are our priority. At bioMérieux, we aren’t just selling fast and reliable solutions – our technical team and scientific experts are fully committed to help you get the most out of your equipment, to ensure the continuity of your operations.
From feasibility, to installation and IT integration and training, our entire range of services can be customized to your needs throughout your whole journey with us, focusing on your core business and optimizing your resources.




