BIOFIRE® Mycoplasma
RAPID TESTING BY ANYONE, ANYWHERE, ANYTIME
RESULTS IN LESS THAN AN HOUR
BIOFIRE® Mycoplasma is a fully automated system that provides simple, accurate, rapid in-house mycoplasma detection for use in testing raw materials, in-process samples, and final product release. We offer validation services designed to meet regulatory requirements—from documentation to comprehensive on-site support.


- BIOFIRE MYCOPLASMA
- Overview
- Technology
- Services
- Resources
There's a better way to do mycoplasma testing — you no longer have to send out your testing, wait 28 days for the compendial method, or rely on a molecular biologist with specialized training.
BIOFIRE® Mycoplasma allows virtually anyone to test for mycoplasma anywhere in the facility at any time, with results in under an hour!
Fully Automated ∙ Easy In-House Mycoplasma Testing ∙ Accurate Results Under An Hour
Applications
BIOFIRE® Mycoplasma is a revolutionary approach to mycoplasma testing that brings value to biopharmaceutical manufacturing.
The current rapid methods available are complicated, requiring highly trained personnel and lengthy turnaround times. The BIOFIRE® Mycoplasma solution provides results in less than an hour, is fully automated, and requires only 2 minutes of hands-on-time.
Thanks to its ease-of-use and small footprint, BIOFIRE® Mycoplasma enables at-line testing near sample collection sites, by personnel non-specialized in mycoplasma detection or molecular techniques.
Safeguard your bioproduction procedures, minimize the impact of costs associated with a possible contamination in the factory, and gain valuable time in releasing batches with BIOFIRE® Mycoplasma.
- Cell Banks
- In-Process Controls
- Culture Media
- Release Tests At The Harvest
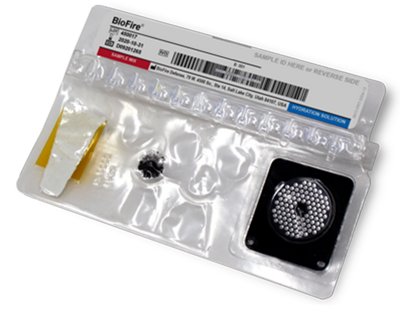
Test Components
The BIOFIRE® Mycoplasma system consists of just two simple components—the FILMARRAY® instrument and the single-use “molecular lab in a pouch” disposable. With these two items, you have everything you need for fast, accurate mycoplasma testing.
Features
- Two minutes of hands-on time
- Fully automated
- Go from sample to result in less than 60 minutes
- Test raw materials, in process samples, and final products
- Detects more than 120 strains of mycoplasma and mollicutes
Compliance
- 21 CFR Part 11 enabled software
- Audit Trail
- User management and access control
- Data Archiving tool
- Audit Trail
Seamless IT Integration
- Our experts are here every step of the way:
- LIMS Connectivity
- Network and Domain integration
- Corporate antivirus compatibility
Services: Feasibility, Validation, and Instrument Support
Your productivity and product quality are our priority. At bioMérieux, we aren’t just selling fast and reliable solutions – our technical team and scientific experts are fully committed to help you get the most out of your equipment, to ensure the continuity of your operations.
From feasibility, to installation and IT integration and training, our entire range of services can be customized to your needs throughout your whole journey with us, focusing on your core business and optimizing your resources.
BROCHURE - Creating Value by Harvesting the Full Potential of your Bioprocessing Testing
DISCOVER OUR COMPREHENSIVE PORTFOLIO
- Filename
- BMX_Bioproduction_Brochure_A4_vF_web.pdf
- Size
- 7 MB
- Format
- application/pdf






