What are Respiratory Tract Infections?
Respiratory tract infections (RTIs) could be classified into two categories, based on the location of the infection: upper respiratory tract and lower respiratory tract.
Upper Respiratory Tract Infections:
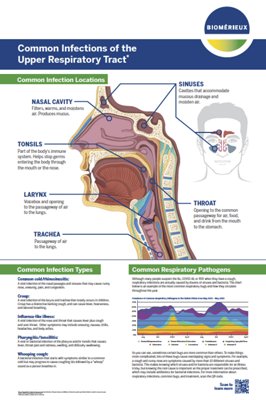
Upper respiratory tract infections (URTIs) affect one or more anatomical regions: ears (otitis), sinuses (sinusitis), throat (angina), and nasopharynx (nasopharyngitis). The main viruses identified in these infections are Rhinoviruses, Coronaviruses, Respiratory Syncytial Viruses, Influenza Viruses, Parainfluenza Viruses, Adenoviruses, Cytomegaloviruses, Epstein Barr Viruses, and Herpes Simplex Viruses, Human Metapneumovirus, and Enteroviruses.
URTIs are very common and are usually caused by viruses that heal without antibiotic treatment. In this case, the antibiotics have no effect, are unnecessary, or even harmful. Treatment then relies on relieving the patient's symptoms.
However, it is recommended to prescribe an appropriate antibiotic in certain clinical situations suggesting a bacterial infection. This is the case of acute purulent otitis media, certain sinusitis, and certain tonsillitis such as, for example, streptococcal angina.
Lower Respiratory Tract Infections:
Potentially more severe than URTIs, lower respiratory tract infections (LRTIs) include acute bronchitis, pneumonia, exacerbation of chronic bronchitis, and bronchiolitis (in young children in general.)
Acute bronchitis is defined as acute inflammation of the bronchi and bronchioles. In the majority of cases it is caused by respiratory viruses including Influenza, Parainfluenza, Adenoviruses, and Respiratory Syncytial Viruses.
Pneumonia is an infection of the lung parenchyma. In community-acquired pneumonia (CAP), bacteria are mainly identified as the source. Streptococcus pneumoniae and Haemophilus influenzae are at the top of the list and atypical bacteria can also be found. These bacteria are frequently associated with secondary infections following a viral respiratory infection.
First-line antibiotics are often effective in treating patients with exacerbated chronic bronchitis or pneumonia, the underlying cause is usually bacterial. However, antibiotics are not effective for lower respiratory tract infections caused by a virus.
What are the healthcare challenges with Respiratory Tract Infections?
Upper respiratory tract infections are the leading cause of disease incidence worldwide. In 2019, 17.2 million episodes occurred, representing more than 2.25 episodes per individual per year (even 3 in children). They are associated with a significant socio-economic burden: it is a major cause of consultation with general practitioners and a major cause of sick leave.1
Despite a decrease in mortality, lower respiratory tract infections are the leading cause of death from infectious disease.2
Taken as a whole, respiratory diseases are responsible for 2.377 million deaths worldwide each year. In industrialized countries, respiratory infections are the main cause of pediatric consultation, are also involved in healthcare-associated infections (HAI), and epidemics.2
The COVID-19 Pandemic
What are the clinical challenges with Respiratory Tract Infections?
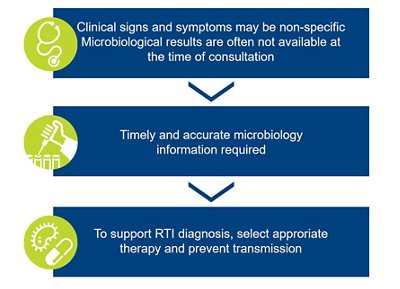
Although very frequent, RTIs remain a challenge for clinicians as they have various clinical presentations / severities and are caused by numerous microbiological agents (bacteria, virus, fungus, parasite).
The clinical assessment is frequently not sufficient to predict the etiological agent, for LRTI. Timely and accurate diagnosis is critical to select the appropriate therapy.
| The clinical assessment is frequently not sufficient to predict the etiological agent, for LRTI. Timely and accurate diagnosis is critical to select the appropriate therapy. | |
|---|---|
| ⬇️ | |
| The clinical assessment is frequently not sufficient to predict the etiological agent, for LRTI. Timely and accurate diagnosis is critical to select the appropriate therapy. | |
| ⬇️ | |
| The clinical assessment is frequently not sufficient to predict the etiological agent, for LRTI. Timely and accurate diagnosis is critical to select the appropriate therapy. | |
What are the Syndromes and Causes of Respiratory Tract Infections?
Upper and lower RTI can be caused by different pathogens. The sample types used to detect these pathogens are different: Upper respiratory tract pathogens (viral and bacterial) are found in nasal, nasopharyngeal and throat specimens and lower respiratory tract pathogens are found in sputum and bronchoalveolar lavage specimens. bioMérieux has solutions to address the 2 types of RTIs and the many infectious agents that cause those infections.
Identification of the pathogen(s) and accuracy of the diagnosis is critical. Many of the pathogens responsible for RTIs are fastidious and require specific conditions for growth. The Targeted Treatment Regimen (TTR) could elucidate the reasons behind the limited prescription rates. The syndromic approach marks a significant breakthrough in improving patient care and facilitating appropriate antibiotic treatments. Conventional microbiology continues to play a crucial role in optimizing therapy and monitoring resistance over time.
COVID-19 Respiratory Co-Infections
Respiratory Tract Infections - Our Diagnostic Offer
As a leader in infectious disease diagnostics, you can count on bioMérieux to deliver the products you need to support RTI diagnostics from sample collection to guided therapy to antibiotic discontinuation. Our respiratory tract infection offer is reinforced by our promise to be a committed service partner for all our products from consultancy to installation, connectivity, training, verification, and customer service to help our customers achieve optimized time to therapy for better patient care and to fight AMR.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
The first step is to assess the risk, collect the culture, and administer empiric therapy. In conjunction with initial clinical assessments, bioMérieux provides the right respiratory tract infection diagnostic tools to aid in the accuracy of early diagnosis and enable more appropriate clinical decisions.
-
Liquid Based Microbiology – LBM®
Universal Tubes for Liquid Sample Collection and Transport
Protect your sample quality with the unique LBM® range featuring a universal tube format for collection and transport, blood culture, sample pre-treatment and enrichment. This universal liquid-based multi-purpose system maintains viability of aerobes, anaerobes and fastidious bacteria for 48 hours.*
LBM® is manufactured by COPAN Company
-
VIDAS® B•R•A•H•M•S PCT™
Important Biomarker for Improved Patient Management
Detects procalcitonin, a biomarker that aids in the risk assessment for progression to severe sepsis and septic shock. PCT also aids in decision making on antibiotic therapy for patients with lower respiratory tract infections (LRTI).
-
VIDAS® KUBE™
Stackable benchtop automated immunoassay solution
VIDAS® KUBE™ is designed to preserve everything labs appreciate about the VIDAS® Solution combined with advanced technology. VIDAS® KUBE™ is a truly flexible, cost-effective automated immunoassay solution providing fast results in complete confidence.
-
BIOFIRE® FILMARRAY® TORCH
Easier testing. Faster results.
Syndromic infectious disease testing with BIOFIRE® FILMARRAY® TORCH is the fastest way to better results.
-
BIOFIRE® Respiratory 2.1 and 2.1plus Panels
1 Test. Up to 23 Targets. ~45 Minutes.
The BIOFIRE RP2.1 and RP2.1plus Panels use the syndromic approach to accurately detect and identify the pathogens most associated with respiratory infections. Fast and comprehensive results may enable better-informed diagnosis and treatment of patients.
-
BIOFIRE® FILMARRAY® Pneumonia & Pneumonia plus Panels
1 Test. Up to 34 Targets. ~1 Hour.
Syndromic tests targeting a comprehensive menu of bacteria and viruses that cause pneumonia and other lower respiratory tract infections, as well as 7 genetic markers of antibiotic resistance.
-
BIOFIRE® SPOTFIRE® System
This Changes Everything.
The BIOFIRE® SPOTFIRE® System is the latest advancement in molecular infectious disease diagnostics from bioMérieux for Point-of-Care testing, offering rapid and actionable results onsite.
-
BIOFIRE® SPOTFIRE® Respiratory/Sore Throat Panels
Comprehensive Onsite PCR Results in ~15 Minutes.
The BIOFIRE SPOTFIRE Respiratory/Sore Throat (R/ST) Panels enable testing for respiratory pathogens from either a nasopharyngeal swab or a throat swab, providing a flexible respiratory solution to meet the unique needs of point-of-care settings.
-
SARS-COV-2 FLUA/FLUB/RSV R-GENE®
Real-time PCR detection kit
Detect & differentiate SARS-CoV-2, Influenza A, Influenza B and RSV in 1 PCR test.
-
PREVI® COLOR GRAM
A Market-Leading Automated Gram Stainer Offering Confidence in Results
Gram staining is a key step in your microbiology workflow, and PREVI® COLOR GRAM is the system to streamline it and provide timely staining results for impactful decisions.
After initial risk assessment and administration of empiric therapy, pathogen detection, identification, and susceptibility testing provide critical information about whether the initial therapy needs to be adjusted. This is especially important because a patient may have an infection that is resistant to certain antibiotics, and because it allows physicians to optimize therapy.
-
WASP®
Automated Microbiology Specimen Processing Instrument
Quality diagnostic results depend on high standard specimen processing. WASP® is the smart solution for truly comprehensive automation – taking you far beyond plating and streaking to address all aspects of microbiology specimen processing.
WASP® System is manufactured by COPAN Company. -
WASPLab®
Integrated Lab Optimization and Automation
The flexible automated specimen processing, incubation, and reading solution that adapts to your unique lab setup and workflow – and even grows with you over time.
WASPLab® System is manufactured by COPAN Company. -
VITEK® MS PRIME
New-Generation Mass Spectrometry Microbial Identification System
VITEK® MS PRIME combines microbiology expertise with innovation taking mass spectrometry to the next level by maximizing the impact of daily laboratory workflow for better patient care.
-
ETEST®
Antibiotic Susceptibility Testing Reagent Strips to Determine On-Scale MICs
Clinicians often need more information than what primary AST can provide. Recognized around the world for their proven performance, ETEST® ready to use reagent strips determine on-scale MICs.
-
VITEK® 2
Fully integrated Identification and Antimicrobial Susceptibility Testing
VITEK® 2, the leading automated system for routine antimicrobial susceptibility testing, provides efficient workflow, faster AST results. Its fully-integrated ID & AST approach ensures superior performance to rapidly and confidently guide therapy.
-
VITEK® 2 ID & AST Cards
Rapid. Flexible. Efficient.
Providing accurate and reliable identification and antimicrobial susceptibility results for clinically relevant organisms with self-contained, disposable test cards designed for VITEK® 2 automated systems.
-
VIDAS® B•R•A•H•M•S PCT™
Important Biomarker for Improved Patient Management
Detects procalcitonin, a biomarker that aids in the risk assessment for progression to severe sepsis and septic shock. PCT also aids in decision making on antibiotic therapy for patients with lower respiratory tract infections (LRTI).
-
MAESTRIA™
New Generation Microbiology Middleware
An innovative, integrated software solution designed to orchestrate your lab routine and transform data into insights.
It is critically important that clinicians assess when to discontinue antibiotics. VIDAS® B•R•A•H•M•S PCT aids in decision making on antibiotic discontinuation for patients with suspected or confirmed bacterial infections.
-
VIDAS® KUBE™
Stackable benchtop automated immunoassay solution
VIDAS® KUBE™ is designed to preserve everything labs appreciate about the VIDAS® Solution combined with advanced technology. VIDAS® KUBE™ is a truly flexible, cost-effective automated immunoassay solution providing fast results in complete confidence.
-
VIDAS® B•R•A•H•M•S PCT™
Important Biomarker for Improved Patient Management
Detects procalcitonin, a biomarker that aids in the risk assessment for progression to severe sepsis and septic shock. PCT also aids in decision making on antibiotic therapy for patients with lower respiratory tract infections (LRTI).
-
VILINK®
Optimize Instrument UPTIME to Improve Laboratory Productivity
A secure solution offering remote access, proactive maintenance, and remote updates. VILINK® diagnoses, solves software and instrument issues, and supports your systems to enable laboratory productivity and efficiency improvements.
-
BIOMÉRIEUX LAB CONSULTANCY
Microbiology Expertise and Consultancy to Optimize Lab Performance
Transforming laboratories together to control costs, maximize quality, shorten time to result, and empower staff.
Useful Resources on Respiratory Tract Infections
Contact us
References
1. Jin X, Ren J, Li R, Gao Y, Zhang H, Li J, et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine. 2021;37:100986.
2. Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious diseases. 2018;18(11):1191-210.