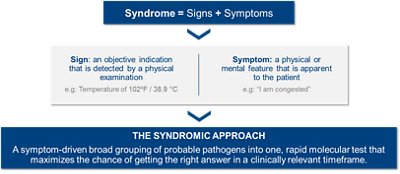
What is the Syndromic Approach?
Determining an infectious pathogen by symptomology alone isn't always possible—infections can present with similar symptoms or even be paired with another, hidden infection. Individually testing just a handful of pathogens may hinder efficient diagnosis and delay optimal patient therapy.
The syndromic approach is a symptom-driven method that groups probable pathogens into one rapid molecular test that maximizes the chance of getting the right answer in a clinically relevant timeframe.
Traditional Testing vs. Syndromic Testing
Traditional Testing
Traditional methods of pathogen identification can be time consuming and lack sensitivity.1

Syndromic Testing
Syndromic testing provides a streamlined workflow and fast, comprehensive results.

What challenges are healthcare professionals facing in terms of infectious disease management?
Healthcare professionals often have to rely on slow, complicated, incomplete, unreliable tests to identify pathogens of infectious diseases and accompanying antimicrobial resistance genes, resulting in suboptimal patient treatment and management.
Clinicians face the following day-to-day challenges:
- Difficulty determining the infectious cause
- The wrong test(s), or no test(s) are ordered
- Increasing costs due to additional testing
- Inappropriate patient care due, in part, to the use of broad-spectrum antimicrobials
- Risk of adverse outcomes and patient dissatisfaction
- Risk of pathogen spread and outbreaks
Additionally, the trend for easier and lower cost access to care has shifted patient care to ambulatory settings. This trend started several years ago but was accelerated during the COVID-19 pandemic. Testing is no longer “lab exclusive” and can occur closer to the patient. Point-of-care testing (POCT) has developed mainly because it is convenient, easy to use, fast and can provide actionable results.2
POCT still has its challenges, especially in the diagnosis of respiratory tract infections (RTIs), which are some of the leading causes of disease worldwide and the most frequent infections among acute illnesses.3 RTIs can be caused by viruses or bacteria, and without a proper diagnosis, unnecessary antibiotics may often be prescribed to patients, leading to the global rise of antimicrobial resistance. While POCT has been developing over the last few years, testing for only 2-3 pathogens may lead to many missed infections and/or co-infections.4 Clinicians cannot rule out co-infections with other respiratory pathogens when diagnosing SARS-CoV-2 infections, nor can they rule out COVID-19 by detecting non-SARS-CoV-2 respiratory pathogens.5
How can our Syndromic Testing Solutions help?
bioMérieux offers syndromic testing solutions for both labs and the point of care.
Syndromic PCR Solutions for Lab Testing with the BIOFIRE® FILMARRAY® TORCH System
The BIOFIRE TORCH System uses multiplex PCR technology to simultaneously test for a comprehensive grouping of targets in about an hour. This is a game-changer for infectious disease diagnostics, arming clinicians with the power of molecular PCR testing with a rapid, easy-to-use approach. BIOFIRE syndromic PCR testing provides proven operational, clinical, and economic benefits with BIOFIRE FILMARRAY Panels that are the right test, the first time for laboratories and healthcare providers.
Rapid Results from Syndromic Testing with the BIOFIRE TORCH System has the potential to:
- Improve timing of patient care decisions.
- Reduce unnecessary or ancillary testing.
- Improve patient outcomes and antimicrobial stewardship.
Syndromic PCR Solutions for Point-of-Care Testing with the BIOFIRE® SPOTFIRE® System:
The SPOTFIRE System is designed to bring central laboratory diagnostic results to the decentralized point-of-care (POC) clinical setting. With a turnaround time of about 15 minutes, SPOTFIRE Respiratory Panels provide actionable answers onsite, which may help inform patient management decisions while optimizing care and antimicrobial stewardship.
Syndromic Testing with the SPOTFIRE System has the potential to:
- Provide fast, accurate, and comprehensive diagnostic results to support clinical decisions.
- Improve patient satisfaction using the right test, the first time to detect multiple pathogens.
- Reduce unnecessary tests, including expensive send-out tests.
Our Syndromic Testing Offer
bioMérieux leads the market in syndromic PCR testing for infectious diseases with our well-established and trusted FILMARRAY technology. The expansion of our syndromic approach at the point-of-care brings syndromic PCR testing even closer to patients to offer a comprehensive molecular portfolio and address all your needs.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
bioMérieux’s latest advancement in syndromic infectious disease diagnostics, the BIOFIRE® SPOTFIRE® System is specifically designed for point-of-care testing. All our expertise and cutting-edge FILMARRAY® technology is at the heart of the system's design. Our revolutionary portfolio of versatile, respiratory PCR tests give clinicians targeted and syndromic options as they care for a variety of patients.
-
BIOFIRE® SPOTFIRE® System
This Changes Everything.
The BIOFIRE® SPOTFIRE® System is the latest advancement in molecular infectious disease diagnostics from bioMérieux for Point-of-Care testing, offering rapid and actionable results onsite.
-
BIOFIRE® SPOTFIRE® Respiratory/Sore Throat Panels
Comprehensive Onsite PCR Results in ~15 Minutes.
The BIOFIRE SPOTFIRE Respiratory/Sore Throat (R/ST) Panels enable testing for respiratory pathogens from either a nasopharyngeal swab or a throat swab, providing a flexible respiratory solution to meet the unique needs of point-of-care settings.
Our automated, user-friendly platforms require just two minutes of hands-on time, optimizing laboratory productivity with automated, around-the-clock diagnostic testing. Features include scalable design, simplified workflow, and LIS-interfacing capabilities. Our panel portfolio includes six syndromic panels providing pathogen-specific results in about an hour. With over 170 targets, BIOFIRE® Panel portfolio offers the largest infectious disease menu on the market.
-
BIOFIRE® FILMARRAY® TORCH
Easier testing. Faster results.
Syndromic infectious disease testing with BIOFIRE® FILMARRAY® TORCH is the fastest way to better results.
-
BIOFIRE® Respiratory 2.1 and 2.1plus Panels
1 Test. Up to 23 Targets. ~45 Minutes.
The BIOFIRE RP2.1 and RP2.1plus Panels use the syndromic approach to accurately detect and identify the pathogens most associated with respiratory infections. Fast and comprehensive results may enable better-informed diagnosis and treatment of patients.
-
BIOFIRE® Blood Culture Identification 2 Panel
1 Test. 43 Targets. ~1 Hour.
The BIOFIRE BCID2 Panel tests for 43 targets associated with bloodstream infections, including Gram-negative bacteria, Gram-positive bacteria, yeast, and 10 antimicrobial resistance genes – all with one test and with results available in about an hour from positive blood culture.
-
BIOFIRE® FILMARRAY® Gastrointestinal (GI) Panels
1 Test. 11 or 22 Targets. ~1 Hour
BIOFIRE FILMARRAY GI Panels are fast and sensitive molecular stool testing methods for infectious gastrointestinal disease.
BIOFIRE® FILMARRAY® Gastrointestinal (GI) Panel tests for 22 targets from one patient sample. Its comprehensive menu includes 13 bacteria, 5 viruses and 4 parasites commonly associated with infectious gastroenteritis.
BIOFIRE® FILMARRAY® Gastrointestinal (GI) Panel Mid tests for 11 targets and offers clinicians another GI testing option. Its menu includes 7 bacteria, 1 virus and 3 parasites associated with infectious gastroenteritis. -
BIOFIRE® FILMARRAY® Meningitis/Encephalitis Panel
1 Test. 14 Targets. ~1 Hour.
The BIOFIRE ME Panel provides answers on 14 of the most common bacterial, viral, and yeast pathogens that cause central nervous system infections to help healthcare providers rapidly distinguish between bacterial and viral meningitis and optimize life-saving therapy.
-
BIOFIRE® FILMARRAY® Pneumonia & Pneumonia plus Panels
1 Test. Up to 34 Targets. ~1 Hour.
Syndromic tests targeting a comprehensive menu of bacteria and viruses that cause pneumonia and other lower respiratory tract infections, as well as 7 genetic markers of antibiotic resistance.
-
BIOFIRE® Joint Infection (JI) Panel
1 Test. 39 Targets. ~1 Hour.
Using PCR technology, the BIOFIRE JI Panel is a rapid syndromic test that delivers comprehensive results in one easy-to-read report.
Providing a comprehensive suite of software solutions that collect, analyze, and manage various sources of BIOFIRE® System data to empower your institution’s decision makers.
-
BIOFIRE® FIREWORKS™
Optimize Diagnostics With Data-driven Solutions.
Providing access to a centralized data management web portal to give users total insights and analytics into BIOFIRE® System performance, utilization, pathogen surveillance, workflow management, and more.
-
BIOFIRE® Syndromic Trends
Turn BIOFIRE® System Results into Smart Pathogen Trending
Creating a global surveillance network that displays BIOFIRE® Panel test results from participating laboratories and hospitals around the world, BIOFIRE TREND allows users to explore epidemiology insights and better understand site-specific and regional pathogen trends and seasonality.
Useful Resources on Syndromic Testing
Contact Us About Syndromic Testing Offer
Contact Us
References
1. Cybulski RJ, et al. Clin Infect Dis. 2018;67(11):1688-96.
2. Nichols JH. Utilizing Point-of-Care Testing to Optimize Patient Care. EJIFCC. 2021 Jun 29;32(2):140-144. PMID: 34421482; PMCID: PMC8343046
3. Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious diseases. 2018;18(11):1191-210.
4. Schreckenberger P, et al. (2015) JCM. 53(10):3110; Data taken from Figure 1
5. Lai C, et al. J of Micro Immuno and Inf. 2020;53(4):505-512. doi:10.1016/j.jmii.2020.05.013 *Contains data from inpatient settings







%20Thumbnail?qlt=85&ts=1706867671952&dpr=off)
%20Thumbnail?qlt=85&ts=1706867672167&dpr=off)