Non-Growth-Based Alternative Sterility Testing
April 07, 2022
SUMMARY
• The SCANRDI® is a non-growth-based rapid microbial method (RMM) that detects not only viable microbial cells that can be isolated using a broth or agar plate but also viable but non-culturable (VBNC) microorganisms, including stressed and fastidious microorganisms that may not be recovered by standard culture methods, making SCANRDI®more sensitive than growth-based methods.
• The FDA has indicated that there are approved drug applications using the SCANRDI® for sterility tests for product release. Therefore the technology is accepted in the pharmaceutical industry.
• It is recognized that some microorganisms will not be subcultured from a scanned membrane for microbial identification purposes due to limitations associated with conventional microbiological media and incubation parameters or exposure to stress associated with the methodology.
• Sample retention strategies should be developed by the end-user that allows them to resample the same material in the event they need to try to recover microorganisms after a positive SCANRDI® result in support of a sterility failure investigation.
• Current Good Manufacturing Practice (cGMP) regulations require that sterility test failures be investigated to determine the most probable cause of the failure. These investigations may be successful without determining the identification of the microorganism.
OVERVIEW
The traditional growth-based sterility test following USP <71> has an incubation time of at least 14 days. That is not suitable for products with a short shelf-life or products prepared for immediate use including compounded sterile preparations (CSPs). In December 2019, the USP <1071> Rapid Microbial Tests for Release of Sterile Short-Life Products: A Risk-Based Approach became official. This general informational chapter provides guidance to the stakeholder on the use of a risk-based approach to the selection of the most appropriate rapid sterility test method for their intended use based on the consideration of the time to result, specificity, the limit of detection (LOD), sample size, product attributes, and patient safety.
As part of the risk assessment, whether a non-sterile drug substance is used in the compounding, the number of aseptic manipulations, the level of environmental control in the compounding facility, and the volume of product injected or infused should be considered in the assignment of the CSP as a low, medium, or high-risk preparation. As a CSP will have a short beyond use dating, the ability to complete the sterility test within 3-4 hours using an alternative Rapid Microbiology Method (RMM), reject microbially contaminated lots, and release passing CSPs to inventory without conducting the 14-day compendial sterility test allows the sterile compounding outsourcing facilities to maintain a sufficient inventory to meet the medical needs and the safety of the patient.
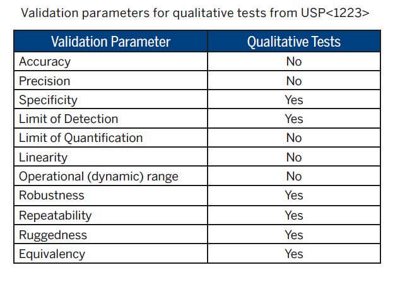
RMMs for sterility testing include both growth-based and non-growth-based methods. Regardless of the type of RMM, all drug products should be evaluated for compatibility with the alternative technology and validated to show comparability to the compendial method by following USP <1223> Validation of Alternative Microbiological Methods. General Informational Chapter <1223> provides guidance on the validation parameters recommended to be performed for a presence/absence qualitative sterility test.
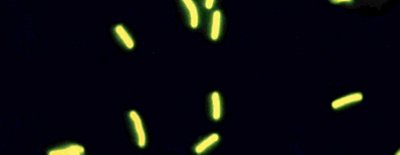
SCANRDI SYSTEM
The SCANRDI® is a solid phase cytometry Rapid Microbiological Method that has been validated meeting the qualitative requirements of USP <1223> and can detect viable single cells of a wide-range of bacteria, yeast, and mold. A description of the technology and the validation details have been submitted to the FDA in the form of a Drug Master File (Type V CDER DMF 014621). In addition, several bioMérieux validation documents are available to companies using the SCANRDI System for testing CSPs. A primary validation report (BMX.1.076552 version 1.0) provides a summary of the data to support LOD, Specificity, Robustness, Ruggedness, and Equivalency. A validation guide (BMX.1.076403 version 1.0) provides method suitability guidance for CSPs.
During sample analysis, SCANRDI® can detect all fluorescent events at the surface of the membrane and recognize labeled microorganisms thanks to the discrimination algorithm. This algorithm is able to reject non-specific fluorescent particles, however, according to the sample matrix, there can still be some non-discriminated events with similar fluorescence characteristics that require analyst verification. Trained laboratory personnel use an incident fluorescence microscope to perform verification. Viable microbial cells are readily differentiated by their shape, size, fluorescence intensity, and fading on longer exposure during fluorescence microscopy to the excitation wavelengths from product-derived auto-fluorescent particles and fibers.
Numerous peer-reviewed publications over the past 25 years have documented the ability of the SCANRDI® System to detect microorganisms in filterable solutions and soluble products. More recent published studies have shown the SCANRDI® System to enumerate viable microorganisms in pharmaceutical grade water, injectable 0.9% sodium chloride, and ophthalmic drug products (1-4). The SCANRDI®technology has been shown to provide consistent and reliable results that are numerically superior and statistically non-inferior to the compendial sterility test with regards to the limit of detection (4). The details of regulatory submissions are confidential, however, the FDA has indicated that there are approved drug applications using the SCANRDI® for sterility tests for product release (5).
The advantages of non-growth-based methods that use Solid Phase Cytometry include:
• Ability to detect stressed and dormant cells that, when cultured traditionally, require longer incubation times, as well as cells with fastidious growth requirements that are often described as viable but non-culturable (VBNC) microorganisms.
• Non-reliance on the limitations associated with the selection and optimization of microbiological growth media and incubation conditions used for the compendial sterility test.
• Avoidance of the delayed recovery of stressed and slow-growing microorganisms that may result in a missed microbial contamination using growth-based methods.
• Ability of the non-growth-based sterility test to be unaffected by antibiotics or other ingredients with antimicrobial activity in the test sample.
• Ability to determine presence/absence of microorganisms in a CSP in as little as 3 hours; allowing for real-time detection and response in the event of an out-of-specification result.
The challenges of non-growth-based methods that use Solid Phase Cytometry include:
• Trained technicians are required for final microscopic verification of events detected by the system. This challenge is overcome by having an internal training program that may include on-site refresher training from bioMérieux and proficiency testing.
• Inability to consistently culture and identify all detected microorganisms post-scanning of the membrane.
CURRENT GOOD MANUFACTURING PRACTICE
FDA’s regulations regarding cGMP requirements for the preparation of drug products have been established in 21 CFR parts 210 and 211 of the Federal Food, Drug, and Cosmetic Act. The FDA released revision 2 of draft guidance for industry in January 2020 on Current Good Manufacturing Practice -Guidance for Human Drug Compounding Outsourcing Facilities Under Section 503B of the FD&C Act (6). The new draft guidance provides conditions that the FDA will not take regulatory action regarding certain cGMP requirements in 21 CFR parts 210 and 211 until new FDA regulations regarding specific cGMP requirements are announced for outsourcing facilities. The primary focus of the draft guidance is on part 211 that relates to the sterility assurance of sterile drug products. The recommendations are consistent with principles of GMP but it also provides a risk-based approach to cGMP requirements.
The 2020 FDA Guidance for Industry states that sterility testing should be conducted using USP <71> Sterility Tests. Any other methods used should be validated and recommends USP <1223> Validation of Alternative Microbiological Methods for general guidance.
OUT-OF-SPECIFICATION STERILITY USING NON-GROWTH-BASED RAPID MICROBIAL METHODS
Benchmarking with customers by bioMérieux has shown that Section 503B Sterile Compounding Outsourcing Facilities and contract laboratories supporting these facilities using SCANRDI® for sterility testing report sterility failure rates of <0.2%. This means that >99% of the time validated product batches are found to be free of microorganisms and can be released for administration within 4 hours. A sterility test failure should be a relatively rare event.
It is a cGMP requirement to conduct an investigation into failed sterility tests to determine the most probable root cause of the failure. Investigations should include batch record review, evaluation of any manufacturing deviations, analysis of environmental monitoring results that include personnel monitoring and trending analysis, confirmation of the efficacy of the environmental controls, and
review of past sterility test failures for patterns. The investigation may also include isolation and identification of the microorganism(s) responsible for the sterility failure to help determine the origin of the microorganism(s) when possible.
In terms of the cGMP-mandated investigation, the end-user could resample the same material and perform the traditional sterility test method or an attempt could be made to try and grow the captured cells post-scan. However, the stress associated with the methodology may prevent the recovery, and incident fluorescence microscopic examination is difficult to be achieved aseptically. bioMérieux has performed a number of studies and have presented an easy post-scan growth method that uses an enriched chocolate agar plate and incubation at 30-35oC for 20-72 hours (7).
The studies were limited to aerobic microorganisms and the data showed good post-scan growth recovery for tested yeast, mold, and gram-positive bacteria. Gram-negative bacteria are the most susceptible to desiccation and are the least likely to be recovered post-scan.
Lack of growth of captured microbial cells does not mean information about the microorganism is completely unknown. The cellular morphology of contaminants observed under the microscope will provide some information to aid the investigation. For example, non-spore-forming rods are most likely to be waterborne while cocci are derived from the human skin. Yeast cells and mycelial hyphal fragments would indicate fungal contamination. Even without the speciation, it is possible to complete a robust investigation and determine the cause of the out-of-specification result.
bioMérieux validation documents available to sterile compounding outsourcing facilities:
• SCANRDI® System Primary Validation Report – Compounded Sterile Preparation: doc no. BMX 1.076552 version 1.0 (Data to support Limit of Detection, Specificity, Robustness & Ruggedness)
• SCANRDI® System Validation Guide – Method Suitability Testing for Compounded Sterile Preparation: doc no. BMX 1.076403 version 1.0
TO DOWNLOAD THE COMPLETE WHITEPAPER, PLEASE COMPLETE THIS FORM
