ARGENE® Ancillary
R-GENE® PCR Kits
Real-time PCR assays for use on open platforms.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
?qlt=85&ts=1706188107875&dpr=off)
?qlt=85&ts=1701291331808&dpr=off)
?qlt=85&ts=1701291347171&dpr=off)
- ARGENE Ancillary
- ARGENE Ancillary
- ARGENE Ancillary
- Overview
- Assays
- Resources
Overview
R-GENE® Assays: Rapid and reliable results for the management of viral infections
Built on more than 30 years of expertise in virological diagnosis, ARGENE® offers a range of reagents, for use on most open real-time PCR systems, for infectious diseases diagnosis.
ARGENE® Expertise
- Simplicity: complete kits, ready-to-use reagents, same pipetting procedure
- Seamless Integration: validated for use on multi-specimens, multi-extraction, and multi-amplification platforms
- Lab Efficiency: common internal control, harmonized extraction and amplification protocols, multiple targeted detection from one extracted sample
Assays
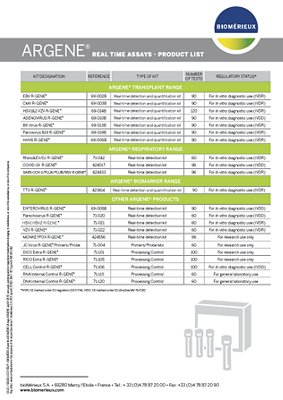
| KIT DESIGNATION | REFERENCE | TYPE OF KIT | NUMBER OF TESTS | REGULATORY STATUS* |
|---|---|---|---|---|
| ENTEROVIRUS R-GENE® | 69-005B | Real-time detection kit | 90 | For In vitro diagnostic use (IVDR) |
| Parechovirus R-GENE® | 71-020 | Real-time detection kit | 60 | For In vitro diagnostic use (IVDR) |
| HSV1 HSV2 R-GENE® | 71-021 | Real-time detection kit | 60 | For In vitro diagnostic use (IVDR) |
| VZV R-GENE® | 71-022 | Real-time detection kit | 60 | For In vitro diagnostic use (IVDR) |
| MONKEYPOX R-GENE® | 424556 | Real-time detection kit | 96 | For research use only |
| JC Virus R-GENE® Primers/Probe | 71-004 | Primers/Probe Mix | 60 | For research use only |
| RNA Internal Control R-GENE® | 71-110 | Processing Control | 60 | For general laboratory use |
| DNA Internal Control R-GENE® | 71-120 | Processing Control | 60 | For general laboratory use |
| DICO Extra R-GENE® | 71-101 | Processing Control | 100 | For research use only |
| RICO Extra R-GENE® | 71-105 | Processing Control | 100 | For research use only |
| CELL Control R-GENE® | 71-106 | Processing Control | 100 | For In vitro diagnostic use (IVDD) |
*IVDR : CE marked under EU regulation 2017/746
*IVDD: CE marked under EU directive 98/79/CE