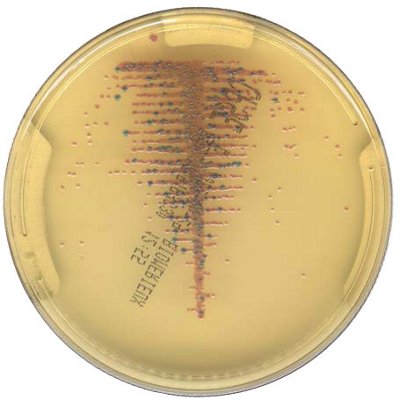
Chromogenic & High Medical Value Media
Patented chromogenic media for rapid, reliable culture & identification for recovery of high prevalence clinically-relevant pathogens
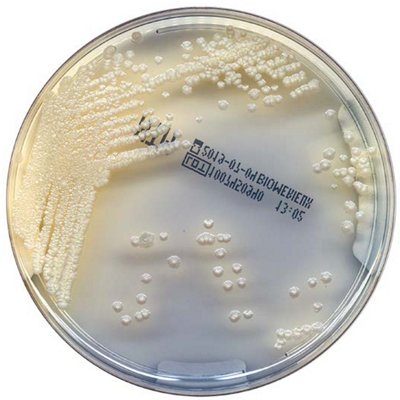
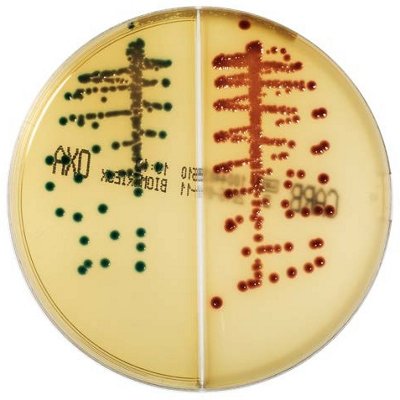
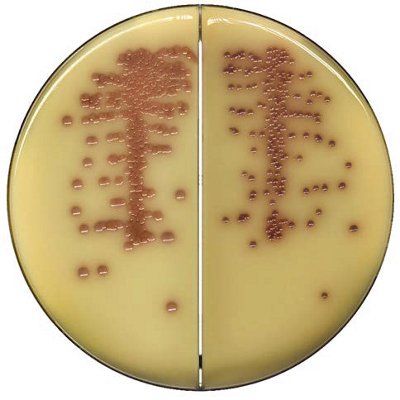
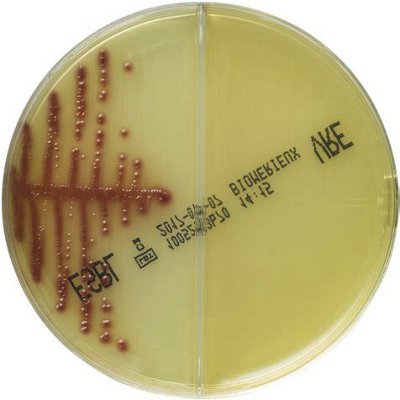
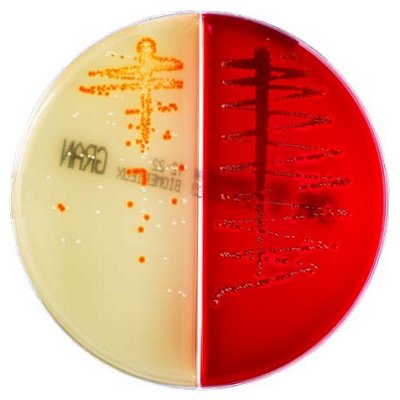
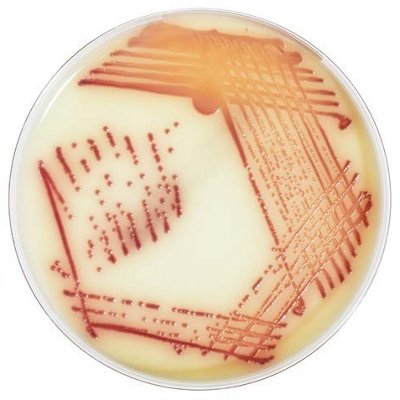
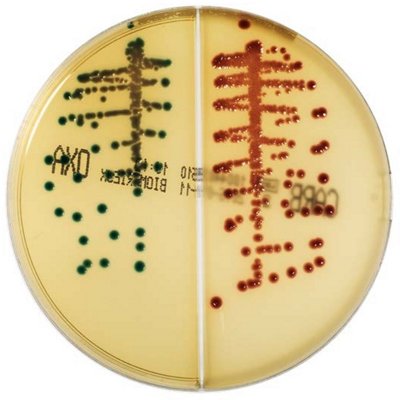
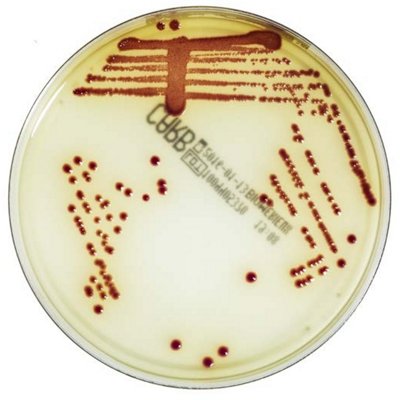
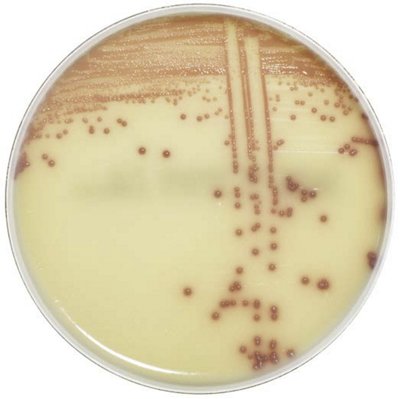
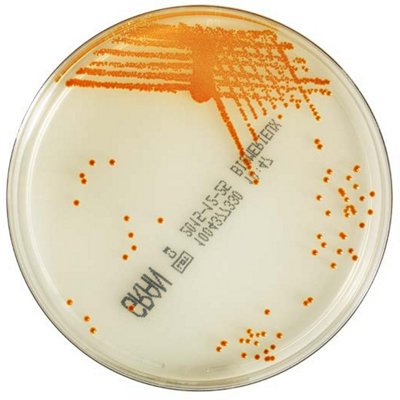
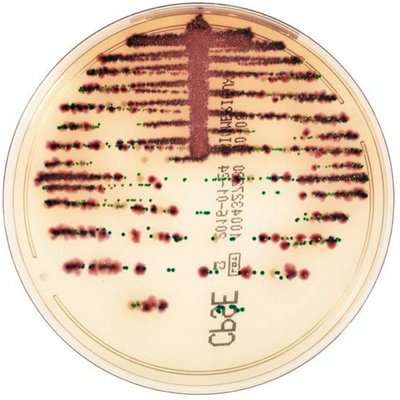
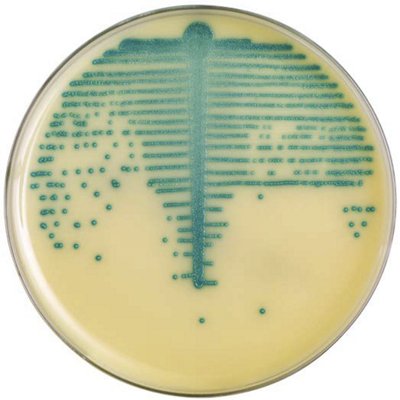
The innovative and extensive CHROMID® range of chromogenic media is the easy way to rapidly and reliably detect and identify microorganisms for faster/direct ID of pathogens and MDRO. The intensity and specificity of the colors of CHROMID® make identification and results clear to see, even with mixed cultures .
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.

- Overview
- Catalog
- Add-on Products
- Resources
Overview
CHROMID® Brings Value to Your Lab
- Easy to read with color intensity and specificity
- Sensitivity and specificity for reliable results
- Fast and actionable results
- Save time with less need for confirmation testing and subculture steps
CHROMID® is Easy to Adopt
- Fast lab staff training with tutorials and reading guides
- Easier reading with intensity and specificity of colors
- Faster/direct identification (ID) and multi-drug resistant organisms (MDRO) detection
- Even mixed cultures are more identifiable than with conventional media
Accelerate Your Results with EARLY READING
CULTURE OF QUALITY BACKED BY CERTIFICATES AND VALIDATIONS FOR CULTURE MEDIA
Quality control (QC) and a secured supply chain processes: confidence during the entire value chain
- Shelf-life and storage temperature: The stability of many of our culture media is validated with thermal shock validation in a reallife lab situation. It allows storage of 2-4 weeks at room temperature (15-25°C) in daily routine use.
- Thermal shock validation: Possible temperature variation accounts during delivery/transportation. Our media are validated using thermal shock simulation sequences. It guarantees the best performance regardless of the shipment conditions.
- The hidden part of the quality certificate: Guarantees consistent product quality for batch-to-batch reproducibility and enables your lab to avoid biological controls, e.g. fertility with QC strains and batch-to-batch comparison, before use.
- High confidence - proven performance: Instructions for Use (IFU) can be used as proof to mitigate risk analysis within your quality management system. Detailed peformances limit the need for heavy validation with biological testing.
- Secured supply chain process: A robust supply chain process guarantees the best delivery times, with an optimized residual shelf life at the customer site for each culture media to guarantee lab staff satisfaction.
- Fast lab staff training: Easy qualification management with reading guides and tutorials.
Unique certificates of compatibility
bioMérieux verifies the compatibility of its culture media with its reagents and systems and issues a validated Certificate of Compatibility, allowing you to gain confidence in compatibility while lightening your workload. You can view and download these certificates at resourcecenter.biomerieux.com.
Catalog
NTM Elite AGAR
- No prior decontamination of the sample is needed (streamline the workflow)
- Usable in routine BSL2 microbiology laboratories
- Incubation at 30°C (enhances the growth of NTM without allowing the growth of BSL3 mycobacteria)
- Fertility: 95% versus 40% with Reference Method (LJ+MGIT)
- Selectivity: 85.6% versus 18.6% with Reference Method (LJ+MGIT
- Compatibility with VITEK® MS
Chronic Pulmonary Disease
Chronic Pulmonary Disease is the most important clinical manifestation of NTM infections. The incidence of pulmonary NTM is increasing. NTM can be found in 30% of patients with pulmonary disease. More than 3 million people died of Chronic obstructive pulmonary disease in 2015.
Cystic Fibrosis
Cystic Fibrosis patients have a high prevalence of NTM infections, and NTM has emerged as a major threat. International guidelines state that culture for NTM should be performed annually in these patients and the need to take precautionary measures to limit transmission of NTM in CF clinics
The ASM and the UK CF trust, say that NTM Elite agar can be utilized for the selective recovery of NTM from respiratory specimens of cystic fibrosis patients.
Identification
Urine Samples
Add-on Products
Atmosphere Generators for Fastidious Bacteria
GENbag and GENbox facilitate culture of bacteria that require anaerobic, microaerophilic or capnophilic atmospheres. This bacterial growth can be observed without opening either system.
GENbag
FOR INCUBATING FROM 1 TO 5 PLATES
NOTE: each GENbag kit contains 20 bags + 20 gaz generators + 2 clip seals.
GENbox
FOR INCUBATING MULTIPLE PLATES
NOTE: each GENbox kit contains 10 gaz generators.