VIDAS® TB-IGRA
Reliable and fully automated solution for Tuberculosis (TB) infection detection
Available on the VIDAS® 3 immunoanalyzer, VIDAS® TB-IGRA is an aid in the diagnosis of Mycobacterium tuberculosis infection.
- image
- Overview
- Assays
- Resources
Overview
TAKE CONTROL OF YOUR TB-IGRA testing with VIDAS® 3
Easily improve your workflow and save time - Detect more TB-infected individuals*
Adequate diagnosis and treatment of Latent Tuberculosis Infection (LTBI) is one of the critical components of the World Health Organization’s End TB Strategy, essential to prevent the development of Active Tuberculosis disease and stop the spread of Tuberculosis.
Our TB Interferon-Gamma Release Assay, VIDAS® TB-IGRA, offers a simple, efficient, and reliable solution to perform in-house diagnosis of TB infection.
Optimize your workflow with an easy TB-IGRA testing solution and save time*
ALL STEPS MANAGED INSIDE VIDAS® 3 from a single whole blood tube
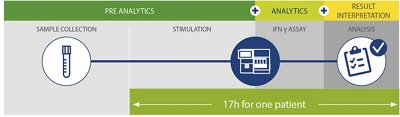
Gain in efficiency by managing TB-IGRA in-house
- Low tech time:
- No multiple tubes to handle
- No sample preparation, no centrifugation
- Simply load & start
- Quick time to Results: Results available in 1 day
- Cost-effective solution: All materials included in the kit
- Good analytical precision*:
- The repeatability estimates (CV%) < 10.0%.
- The reproducibility estimates (CV%), i.e. total between-lot between-instrument precision, < 15.0%
Detect more TB-infected individuals*
Provide reliable results for TB infection
- Excellent and enhanced sensitivity: 97.5% in active TB population**
- High confidence in positive results on patients at high risk for TB infection
- High specificity performance (97.6%) on population at extremely low risk level of TB exposure.
- Strong agreement on population with mixed risk of TB infection with comparable solution*
- Less indeterminate results*
VIDAS® Solutions
- Reliable and easy-to-use instruments with random access and small foot-print.
- Well adapted to rapid response laboratories.
- Factory-calibrated, single-dose tests which reduce the need for additional controls.
- Short time to result.
* Compared to QuantiFERON®-TB Gold Plus test (QFT®-Plus) - See PERFORMANCE tab for further details
** Compared to the gold standard: culture
Ref: VIDAS® TB-IGRA Package insert 053331-04
CE Marked. Other health authorities approvals pending
VIDAS® TB IGRA Video
VIDAS® TB IGRA Video
CE Marked. Other health authorities approvals pending
Assays
Technical Specifications
| Assay | Reference | Code | Tests per Kit | Time to Result | Decisional Cut-Offs |
|---|---|---|---|---|---|
| VIDAS® TB-IGRA | 423111 | TBRA | 60 | 17 hours | Positive, Negative, indeterminate |
| VIDAS® IFNg QC PANEL | 424069 | IFNg | N/A | 43 minutes | N/A |
BECAUSE IT MAKES SENSE ON VIDAS®